Background: Acute myeloid leukemia (AML) blasts often highly-express IHH, GLI1, GLI2, and other elements of the Hedgehog (HH) pathway, which induces downstream pro-survival gene expression. HH pathway overactivation in AML blasts is associated with chemoresistance in vitro and ex vivo and has associated with worse survival in post-hoc clinical analyses.
Glasdegib is an oral antagonist of the HH pathway activating element Smoothened (SMO) that is able to stall cell-cycle progression of AML leukemic stem cells in vitro and ex vivo. Glasdegib in combinations with low-dose cytarabine (LDAC) is a recent FDA-approved option for intensive therapy-ineligible patients (pts). However, LDAC is not often used in the United States and may not be the optimal glasdegib partner, which may be a hypomethylating agent (HMA) like azacitidine (AZA). We previously-presented the favorable clinical trial data for the combination of glasdegib and AZA in older and mostly ELN poor-risk disease pts (NCT02367456). The HMAs AZA and decitabine (DEC) are related, but not equivalent. Many hematologists prefer DEC for different reasons including experience, shorter therapy duration when given over 5 days (DEC5), and insurance coverage. Ten-day decitabine (DEC10) has shown promising activity in pts with AML, specifically TP53-mutated disease. Based on promising early data for glasdegib in combination with DEC5, the possibility of favorable responses with DEC10 in pts with poor-risk AML, and the experience that some intensive therapy-ineligible pts cannot accept the risks of myelosuppression and toxicity with prolonged venetoclax (VEN) administration, we proposed a study of glasdegib in combination with either DEC5 or DEC10 for pts ineligible for or not willing to proceed with intensive therapy or HMA-VEN.
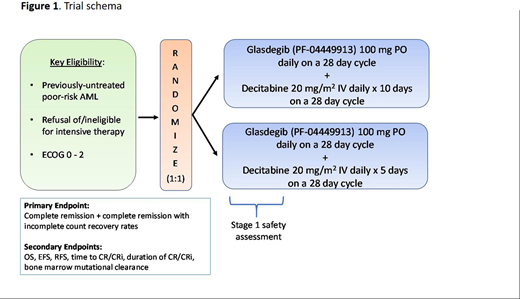
Study Design and Methods: GLAD-AML is a multi-center, randomized phase 2 study (NCT03798678) designed to evaluate the efficacy of glasdegib in combination with either DEC5 or DEC10 in pts with newly-diagnosed poor-risk AML who either refuse or are ineligible for intensive therapy. Key inclusion criteria are a morphologically-confirmed diagnosis of AML according to WHO 2016 classification with poor-risk disease as defined by the cytogenetic or molecular abnormalities (excluding FLT3-mutated AML). Key exclusion criteria are prior therapy for myeloid malignancy as well as candidacy and willingness to receive intensive therapy. Pts are randomized 1:1 to receive wither DEC5 or DEC10 in combination with glasdegib, which is administered at the starting dose of 100 mg orally once daily and continuously on 28-day cycles (Figure 1).
A Simon two-stage design will be employed. Assuming a historical CR rate of 17% for DEC5 based on the DACO-16 trial, a minimum acceptable CR rate of 25% (given the possible additional toxicity from glasdegib) and a predicted minimum CR rate of 47% (the alternative hypothesis) for pts accrued to either arm, a minimum of 23 evaluable pts per arm will be accrued to test for a statistically-significant difference with an 80% power and a 90% confidence interval (a type 1 error rate, alpha one-sided of 0.05). The primary objective of this study is to evaluate the toxicity and safety profiles of glasdegib/DAC5 and glasdegib/DAC10. Other secondary objectives include determining EFS, RFS, OS, duration of response and mutation clearance. Intended exploratory endpoints include the measurement and detection of any changes in the amount of bone marrow aspirate mRNA expression or protein quantification of the Hh pathway-related proteins SMO, GLI1, and GLI2 via mRNA isolation with reverse transcription-polymerase chain reaction and monoclonal antibody-assisted immunohistochemistry, respectively.
The proposed study will "pick the winner" among the two above-mentioned therapies as a step towards establishing a new, more effective standard-of-care for AML pts with poor-risk disease including therapy-related and TP53-mutated AML who are ineligible for intensive therapy and risk averse to VEN combination therapy. The trial is ongoing. The work was in part supported by the DeLuca Center for Innovation in Hematology Research at Yale Cancer Center and The Frederick A. Deluca Foundation.
Podoltsev:Incyte: Consultancy, Honoraria; Agios Pharmaceuticals: Consultancy, Honoraria; Blueprint Medicines: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Genentech: Research Funding; AI Therapeutics: Research Funding; Kartos Therapeutics: Research Funding; Alexion: Consultancy, Honoraria; Bristol-Myers Squib: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Astex Pharmaceuticals: Research Funding; Boehringer Ingelheim: Research Funding; Astellas Pharma: Research Funding; Daiichi Sankyo: Research Funding; Sunesis Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Research Funding; CTI biopharma: Consultancy, Honoraria, Research Funding; Samus Therapeutics: Research Funding; Arog Pharmaceuticals: Research Funding. Prebet:Jazz Pharmaceuticals: Consultancy, Research Funding. Zeidan:Celgene / BMS: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Otsuka: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; Taiho: Consultancy, Honoraria; ADC Therapeutics: Research Funding; Aprea: Research Funding; Astex: Research Funding; Daiichi Sankyo: Consultancy, Honoraria; Cardinal Health: Consultancy, Honoraria; Trovagene: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; MedImmune/Astrazeneca: Research Funding; BeyondSpring: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria; Acceleron: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Leukemia and Lymphoma Society: Other; CCITLA: Other; Cardiff Oncology: Consultancy, Honoraria, Other.
The abstract presents the rationale for the use of glasdegib, a Hedgehog pathway inhibitor, in combination with decitabine, a combination that is not FDA approved at present.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal